The certification behind Health Check
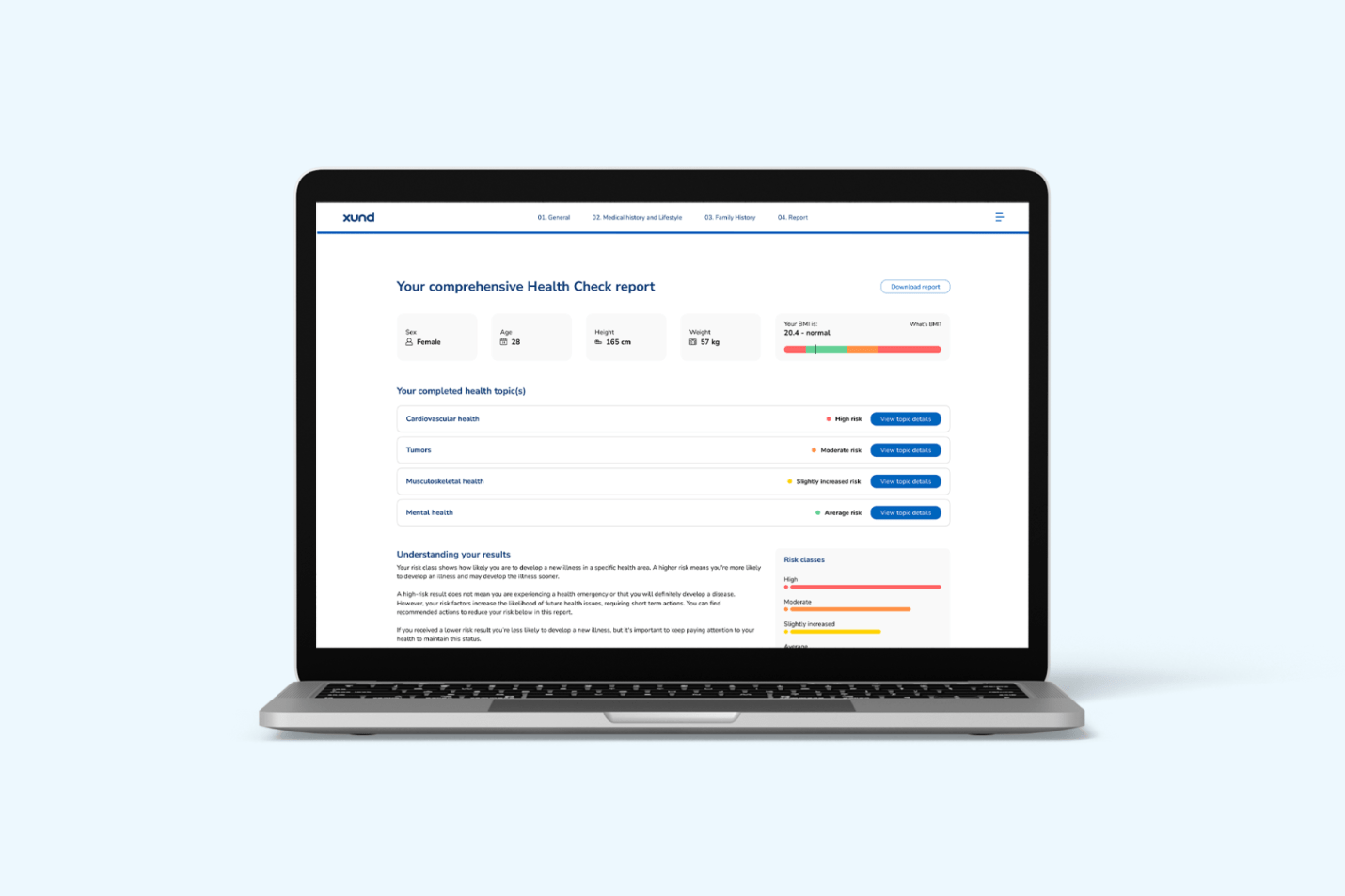
With Health Check, we're introducing a powerful prevention tool designed to provide health-conscious or asymptomatic users with a comprehensive 360-degree risk evaluation. The outcome is a personalized risk profile across 14 health topics (with 3 available as of now), along with tailored recommendations to help users take preventive action and avoid costly health issues down the road. As a certified medical device under the Medical Device Regulation (MDR), Health Check meets rigorous regulatory standards to ensure safety and reliability.
We spoke with three key experts at XUND: Sophie Pingitzer (Head of Quality), Mark Toth (Head of Product), and Peter Mendik (Medical Expert), to better understand the certification process behind Health Check. Here’s what they shared.
Can you describe Health Check in a few sentences?
Mark: Health Check helps users understand their individual risk of developing diseases of a specific health topic (e.g., cardiovascular health). By answering targeted questions about their lifestyle, demographics, medical history, family history, and more, users receive a validated and personalized health risk assessment as well as recommendations on how to tackle their individual risk factors.
Sophie: As of now, we have three health topics available – mental health, cardiovascular health, and tumors – with eleven additional topics on the way. From a regulatory perspective, Health Check is classified as a class I Software as a Medical Device (SaMD) under the MDR.
Could you provide an overview of the certification process? How does it typically work for a class I SaMD?Sophie: The essential requirements are the same for all classes of medical devices: We have put together a technical documentation which shows that Health Check is safe and effective. This includes an extensive description of software development and testing, risk management to properly address patient risks, usability engineering to ensure the product can be correctly used, and a clinical evaluation to demonstrate the medical quality. With class I products, this evidence serves as the basis for the CE marking.
What are the key differences between class I and class IIa medical devices?
Mark: In short, the risk the device poses to the user is a major factor in the class it is assigned. So, class I devices are considered to have a lower risk level.
Sophie: This also means that class I devices do not require the involvement of a Notified Body, unlike medical devices of class IIa such as our Symptom Check or Illness Check.
What benefits does this certification bring to Health Check and its users?
Mark: For users, the certification provides confidence that Health Check delivers reliable and clinically validated insights into their health, similar to what they would receive from a preventive checkup with a doctor. As part of the certification process, the risk results of Health Check were compared to the analysis performed by actual doctors, ensuring that its results are aligned with professional medical evaluations. This enhances the product’s trustworthiness, benefiting both the manufacturer and users.
Peter: Essentially, what users get with the certified Health Check, is a validated tool that offers a level of reliability and performance data that fitness or lifestyle apps typically lack.
What challenges did you face during the certification process, and how did you approach them?Sophie: The most difficult evidence to produce was the clinical evaluation. Due to the novelty of the product, there is no obvious way to measure the quality of the risk assessment and no obvious benchmark to compare it to. As the logical next step, we conducted a systematic literature review and concluded from the data that, for the purpose of Health Check, the state of the art remains expert opinion rather than digital tools. Furthermore, there is no universal definition that holds across any available tools and medical literature. These were challenges we needed to address during the design and development of the product, which we eventually managed to overcome successfully.
Can you explain the clinical evaluation that was needed for Health Check?Sophie: In order to show that Health Check can reliably assess the health risks of individuals, we needed a comprehensive, real-life dataset that follows patients over many years and tracks medical history, lifestyle choices, and so on. We managed to obtain such a dataset via the UK Biobank, which is an impressive, ongoing study producing exactly this kind of data. Purely getting access to the data was a challenge, and then further processing it to make sure it could be used properly for our project was another.
Peter: The clinical evaluation was essential because we weren’t just creating a lifestyle app – we were developing a validated medical device. To achieve this, the real-world data had to be highly specific to ensure reliability. This approach allowed us to measure actual clinical outcomes and assess the performance of our product. Unlike most clinical risk assessment tools that focus on specific endpoints, we envisioned a solution that could capture the bigger picture of patients’ overall health.
What major cross-team collaborations were needed to achieve market release?Sophie: The design and development of Health Check was a cross-functional effort from the start. This involved medical experts, developers, data scientists, UI/UX designers, and the regulatory team, who collectively ensured that all requirements to build a safe and effective product were fulfilled. Bringing in these different perspectives was crucial to the success of the project.
Mark: Releasing Health Check as a medical device required significant cross-team collaboration across several functions. One major area of collaboration is balancing usability with medical accuracy. This needs constant alignment between teams to create a product that’s user-friendly while maintaining the highest medical standards. Another key area is the development and refinement of the risk model which demands close cooperation across functions to evaluate performance against real-world data and adjust it if needed after generating the results.
What advice would you offer to companies looking to bring medical devices to market?Sophie: Build a culture of quality within the team and be conscious of all regulatory requirements from the start – this will save you work down the line and ensure compliant development of the product. Don’t underestimate the efforts that go into building a medical device, whatever the risk class.