Roche
In September 2023, Roche Diagnostics and XUND launched a project to assess the risk of heart failure in patients with a history of COVID-19 infection. The aim of the collaboration is to use XUND's platform-agnostic technology to improve patient access and identify at-risk patients earlier, thereby reducing hospital admissions and mortality.

About Roche
Since its founding more than 125 years ago, Roche has become a major player in the healthcare sector. Always striving to be at the forefront of innovation, the company has been actively engaging in research and development of diagnostics and pharmaceuticals. Its division, Roche Diagnostics, operates in more than 100 countries. Their primary focus is on advancing diagnostic solutions that empower healthcare professionals to make critical decisions, and improving ways to enhance the seamless integration of these tools into global healthcare systems in order to achieve optimal outcomes.
Modules
- Illness Check
Add-ons
- Ecosystem Management
- User Insights
Regions
- Germany
Languages
- English
- German
Project overview
Roche and XUND launched a collaborative partnership in response to the repercussions of the COVID-19 pandemic on both individuals and the healthcare systems worldwide.
With 2.5 million patients suffering from heart failure as of 2022, it is one of the most costly and deadly diseases in Germany. Traditional laboratory testing is usually deployed too late in the progression of disease due to a lack of proper screening. This delay results in a high number of severe cases, many of which could be preventable, leading to high costs and capacity constraints on the healthcare system.
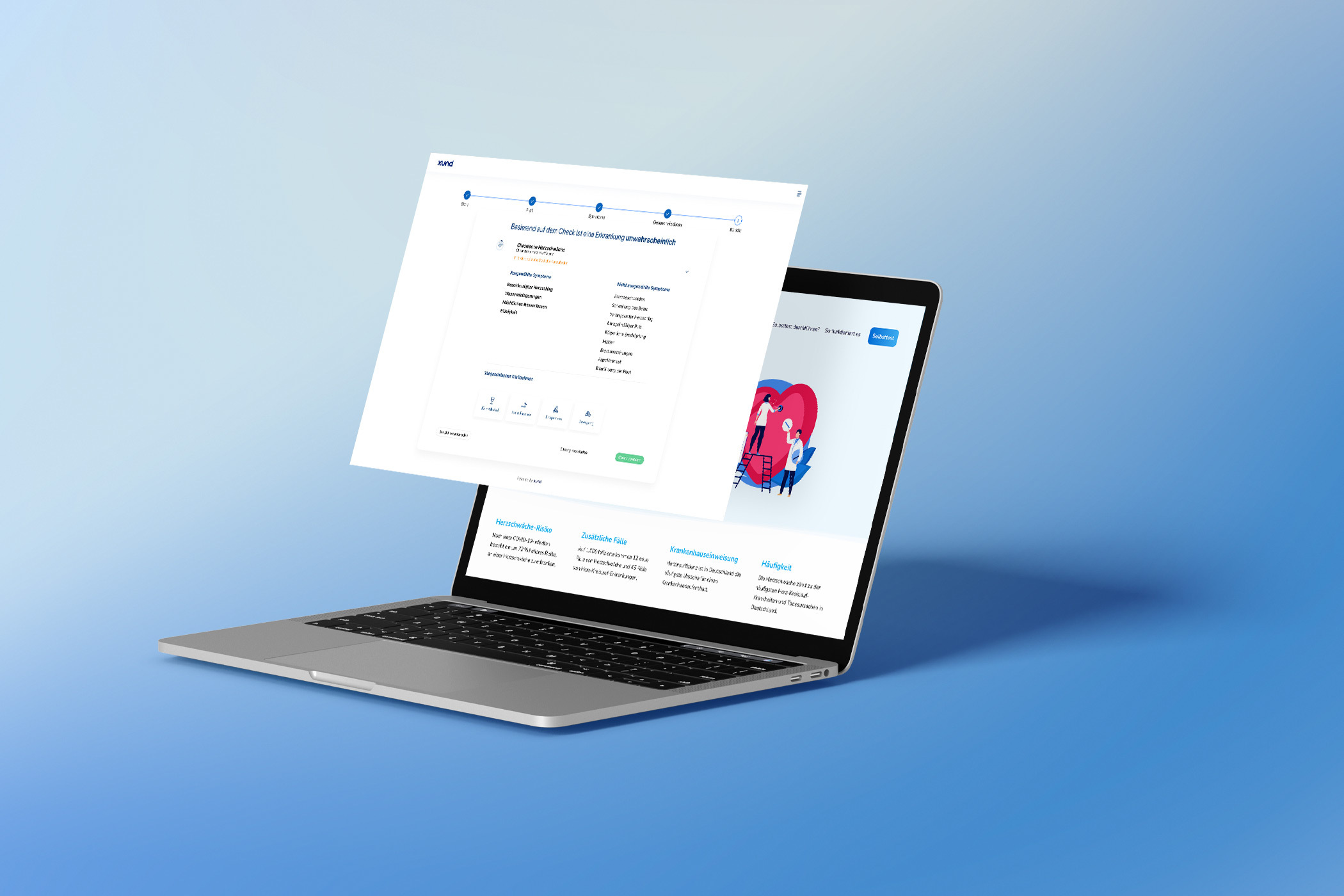
In the aftermath of the pandemic, while the long-term outcomes are still uncertain, ongoing research suggests a 72% increased risk of heart failure following recovery from COVID-19. For every 1,000 infected patients, there are 12 more cases of heart failure and a total of 45 additional cases of any of the 20 investigated cardiovascular diseases.
To prevent high mortality rates, new systems need to be implemented to identify people at higher risk of heart failure earlier. Medical technology, such as XUND's Patient Interaction Suite, can provide an answer to this challenge by providing easy access to trusted and reliable risk assessments, enabling scalable patient screening for specific risks far beyond current capabilities.
Outcome
Through this collaboration, the project partners aim to improve early detection of heart failure following a COVID-19 infection, prevent cases from going untreated, reduce hospital admissions, lower treatment costs, alleviate the burden on healthcare personnel, and, ultimately, save lives.
The goals of the pilot program are to:
- Offer an easy-to-do risk assessment and raise awareness about the increased risk of heart failure after a SARS-CoV-2 infection.
- Prevent high mortality rates, reduce costs, and address staffing shortages.
- Develop a leading platform for risk stratification that goes beyond cardiology.
“(...) by using AI ....”
XYAA at Roche Diagnostics
Why Roche decided to choose XUND
Roche places a significant emphasis on the value of partnerships. By working closely with external partners, their goal is to develop integrated solutions that deliver greater medical and health advantages. Roche remains consistently tuned into the latest developments, ensuring they are always poised to provide cutting-edge solutions for both current and future challenges.
Following up on this commitment, the collaboration with Roche was launched to leverage the power of XUND's platform-agnostic Patient Interaction Suite and bring early detection of heart failure to the next level.
The flexibility of our API-first Software as a Medical Device (SaMD) enabled seamless integration across multiple digital platforms, providing us with an omni-channel approach to patient access. Furthermore, XUND's certification as a class IIa medical device compliant with EU MDR, along with our comprehensive and proactive approach to regulatory compliance, played a pivotal role in Roche choosing XUND for this collaboration project.